Colloid Vs Suspension Examples . a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the main difference between colloid and suspension lies in the size of particles. See how colloids differ from. learn the distinguishing characteristics of these similar but different types of mixtures. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Colloid particles are much smaller than suspension particles. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability.
from cekjtznh.blob.core.windows.net
the main difference between colloid and suspension lies in the size of particles. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the distinguishing characteristics of these similar but different types of mixtures. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. See how colloids differ from. Colloid particles are much smaller than suspension particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the.
Colloid Compound Solution Suspension at Robert Rutledge blog
Colloid Vs Suspension Examples learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. learn the distinguishing characteristics of these similar but different types of mixtures. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. the main difference between colloid and suspension lies in the size of particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Colloid particles are much smaller than suspension particles. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. See how colloids differ from.
From cegexkfz.blob.core.windows.net
Colloid Solutions Quizlet at Katrina Cohen blog Colloid Vs Suspension Examples learn the distinguishing characteristics of these similar but different types of mixtures. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn the. Colloid Vs Suspension Examples.
From www.pinterest.com
solutionssuspensionsandcolloids9728.jpg (728×546) Science Colloid Vs Suspension Examples a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and.. Colloid Vs Suspension Examples.
From cexalzsn.blob.core.windows.net
Example Of Suspension And Colloid at Blaine Waltz blog Colloid Vs Suspension Examples learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. See how colloids differ from. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. Colloid particles are much smaller than suspension particles. learn the distinguishing characteristics of these similar but different types of mixtures. . Colloid Vs Suspension Examples.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Vs Suspension Examples Colloid particles are much smaller than suspension particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples. Colloid Vs Suspension Examples.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Colloid Vs Suspension Examples a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. learn the. Colloid Vs Suspension Examples.
From www.youtube.com
Solution Suspension Colloid YouTube Colloid Vs Suspension Examples the main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. a colloid is a. Colloid Vs Suspension Examples.
From cexalzsn.blob.core.windows.net
Example Of Suspension And Colloid at Blaine Waltz blog Colloid Vs Suspension Examples learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the main difference. Colloid Vs Suspension Examples.
From pt.slideshare.net
Solutions, suspensions, and colloids Colloid Vs Suspension Examples learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon. Colloid Vs Suspension Examples.
From thecontentauthority.com
Colloid vs Suspension Which One Is The Correct One? Colloid Vs Suspension Examples because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the distinguishing characteristics of these similar but different types of mixtures. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. Colloid particles are much smaller than suspension. Colloid Vs Suspension Examples.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Colloid Vs Suspension Examples See how colloids differ from. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. Colloid particles. Colloid Vs Suspension Examples.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Colloid Vs Suspension Examples a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn the distinguishing characteristics of these similar but different types of mixtures. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. learn the difference between colloid and suspension, two types. Colloid Vs Suspension Examples.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Vs Suspension Examples a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the distinguishing characteristics of these similar but different types of mixtures. the main. Colloid Vs Suspension Examples.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Colloid Vs Suspension Examples See how colloids differ from. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with examples and. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size,. Colloid Vs Suspension Examples.
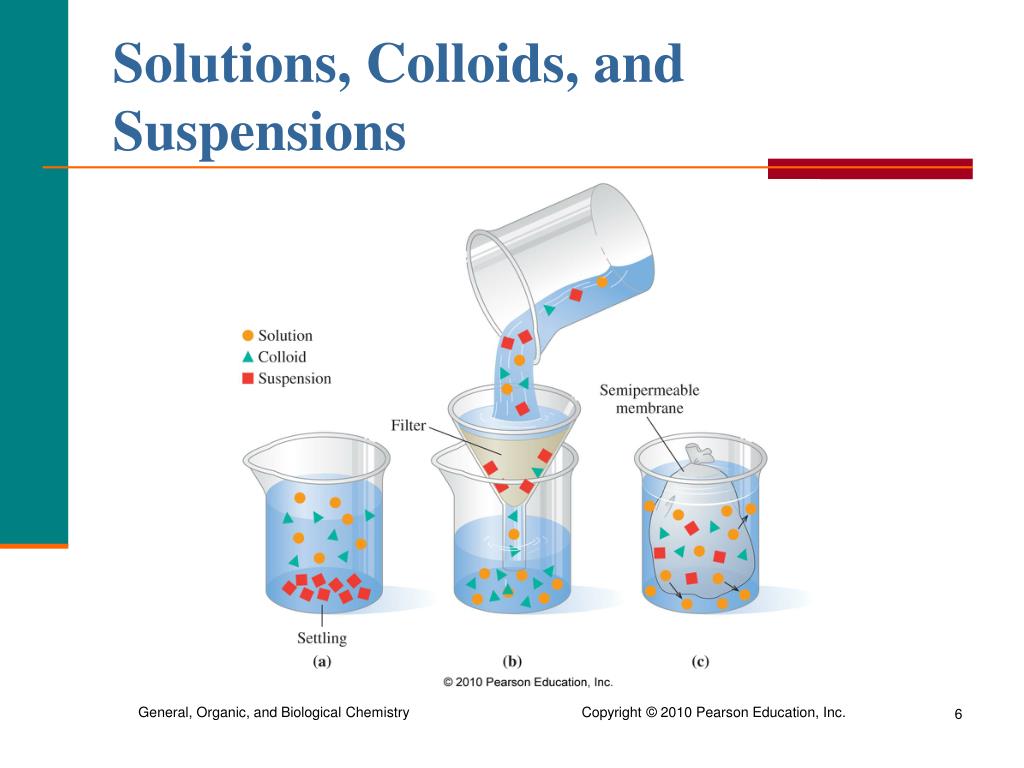
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Vs Suspension Examples learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. See how colloids differ from. learn the distinguishing characteristics of these similar but different types of mixtures. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn. Colloid Vs Suspension Examples.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Colloid Vs Suspension Examples Colloid particles are much smaller than suspension particles. the main difference between colloid and suspension lies in the size of particles. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the difference between colloid and suspension, two types of heterogeneous mixtures, with. Colloid Vs Suspension Examples.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Colloid Vs Suspension Examples Colloid particles are much smaller than suspension particles. See how colloids differ from. because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. learn the definitions, properties, and examples of suspensions and colloids, two types of mixtures with particles of different sizes. learn the. Colloid Vs Suspension Examples.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Colloid Vs Suspension Examples because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Colloid particles are much smaller than suspension particles. learn how to distinguish colloids, suspensions, and solutions based on their composition, particle size, and stability. the main difference between colloid and suspension lies in the. Colloid Vs Suspension Examples.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Colloid Vs Suspension Examples because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. the main difference between colloid and suspension lies in the size of particles. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. learn. Colloid Vs Suspension Examples.